
A substance can be a mixture if it is a combination of two or more substances that are not chemically bonded together and can be easily separated by physical means.\): Definitions of the Atomic Radius. A substance is an element if it is a pure substance and cannot be broken down into simpler substances by chemical means.

How can it be determined whether a substance is a compound or not?Ī substance is a compound if it is made up of two or more different elements chemically bonded together.
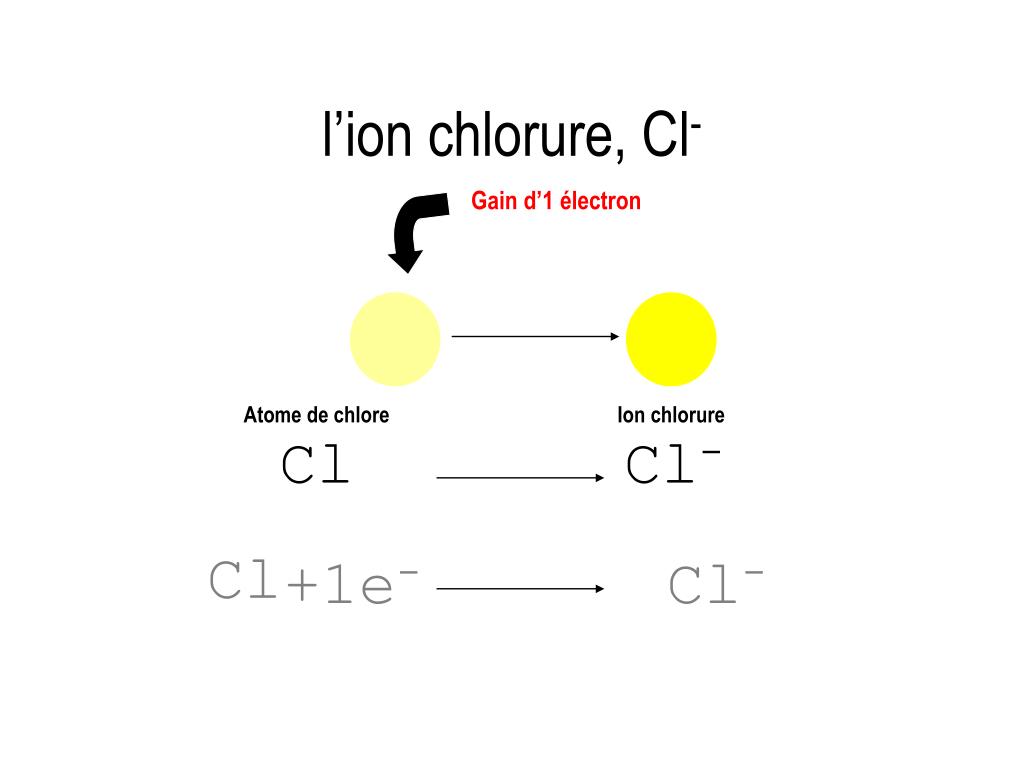
The atoms or ions in the salt are held together by an ionic bond, which is formed by the transferring of electrons from the sodium atom to the chlorine atom, creating positively charged sodium ions and negatively charged chloride ions. What is the chemical bond that holds salt together? It must be broken down through chemical means. The elements in salt cannot be separated by physical means alone. The chemical formula for table salt, which is the most common form of salt, is NaCl. Salt cannot be considered a mixture because it is combined in a fixed ratio.It is specifically an ionic bond because it is formed by the transfer of electrons.The atoms that make sodium chloride are sodium and chlorine atoms. Sometimes, when table salt is combined with other compounds, such as anti-caking agents, it is then referred to as a mixture rather than the salt itself. The chemical bond between the sodium and chlorine atoms creates a new substance, sodium chloride, that has unique properties and cannot be separated by physical means alone. Salt, on the other hand, is made up of two or more different elements chemically bonded together. Can Salt be considered a Mixture?Ī mixture is a combination of two or more substances that are not chemically bonded together and can be easily separated by physical means such as filtration, evaporation, or centrifugation.įor example, salt and pepper are mixtures. This chemical combination forms a new substance, sodium chloride, which is different from either sodium or chlorine individually, and thus, it is not an element.Īlso Refer: Difference between an Element and a Compound 3. Salt is composed of the elements sodium (Na) and chlorine (Cl) that are chemically bonded together in a 1:1 ratio. Instead, the atoms that make sodium chloride- sodium and chlorine can be found in the periodic table. When we look at the periodic table, we don’t find any element named sodium chloride. Why can Salt not be considered an Element?Īn element is a pure substance that cannot be broken down into simpler substances by chemical means. The chemical bond is formed by the transfer of electrons from the sodium atom to the chlorine atom, creating an ionic bond and resulting in the formation of positively charged sodium ions and negatively charged chloride ions.Īlso Refer: Ionic Compounds VS Molecular Compounds 2. The elements sodium (Na) and chlorine (Cl), which are chemically linked in a 1:1 ratio, make up table salt (sodium chloride). 1.1 What makes Salt a Compound?īeing composed of two or more different elements that have been chemically bonded together, salt is a compound.

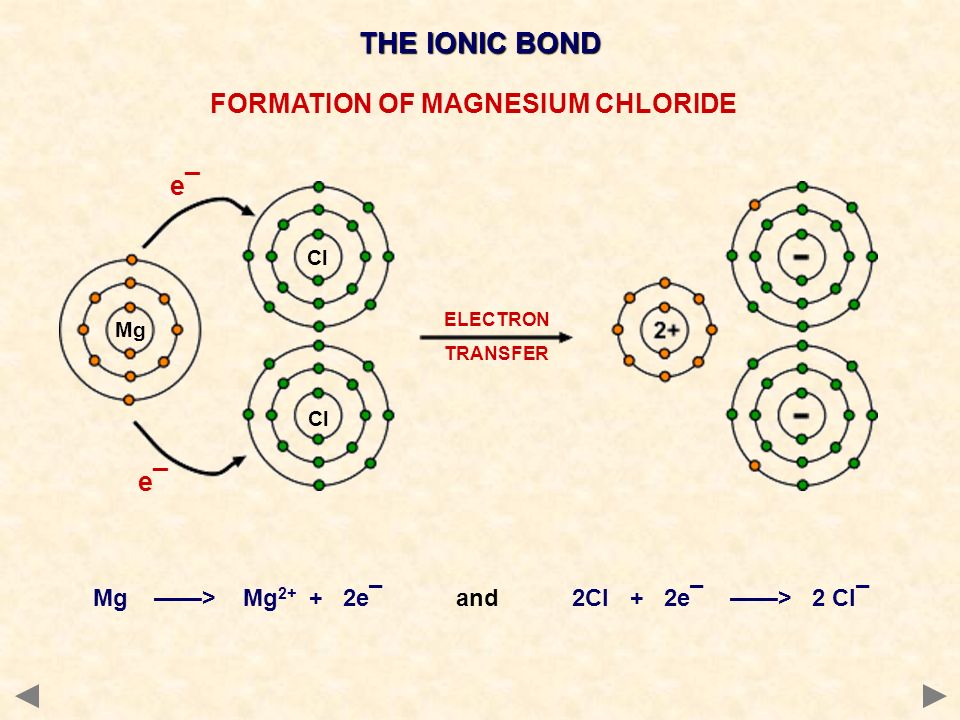
Salt is a compound, specifically a chemical compound composed of the elements sodium and chlorine. These bonds can be formed by sharing or exchange of electrons between atoms. When the elements or ions come together, they interact and create chemical bonds that are difficult to break. A compound is anything formed when two or more different chemical elements or ions are mixed together in a specific proportion.


 0 kommentar(er)
0 kommentar(er)
